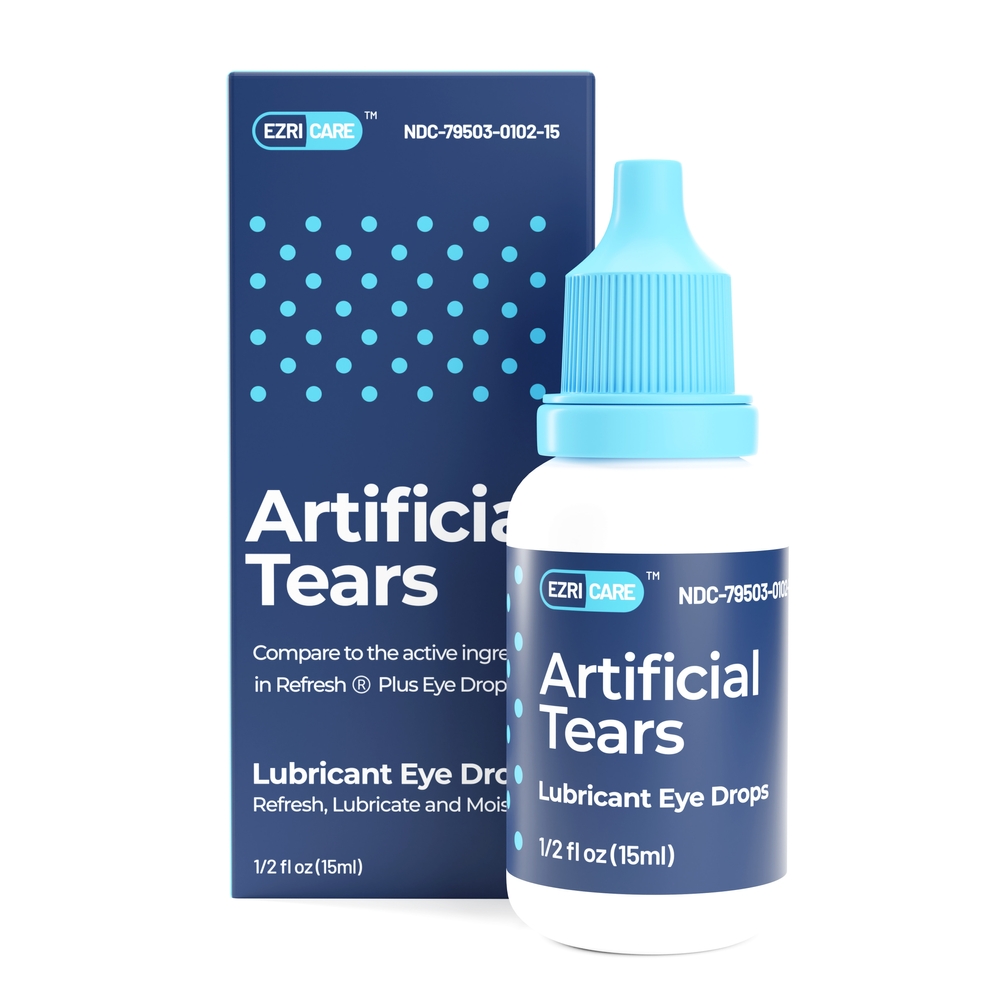
Artificial Tears Eye Drops Lawsuits
About Artificial Tears Lawsuits
- Contaminated artificial tears, specifically EzriCare Artificial Tears and Delsam Pharma’s Artificial Tears and Artificial Eye Ointment, have been linked to bacterial eye infections that can lead to serious health risks, including vision loss and even death.
- As a result of the contamination and the associated health risks, there have been recalls of EzriCare and Delsam Pharma’s artificial tears products.
- With the help of an experienced artificial tears lawyer, victims with permanent vision loss and other injuries from contaminated artificial tears can file a personal injury lawsuit to seek compensation for medical bills, lost wages, pain and suffering, and more.
Artificial Tears Lawsuits FAQs
Is there a lawsuit against artificial tears?
Yes, lawsuits are being filed against the manufacturers and retailers of artificial tears eye drops. Artificial tears lawsuits seek compensation for injuries and damages caused by bacterial infections linked to using contaminated products.
What brands of artificial tears are being recalled?
Global Pharma Healthcare has recalled Artificial Tears Lubricant Eye Drops by EzriCare and Delsam Pharma, and Delsam Pharma Artificial Eye Ointment.
Are there long-term side effects of recalled artificial tears?
The bacterial infections caused by contaminated artificial tears can have long-term side effects, including permanent vision loss. The strain of bacteria, Pseudomonas aeruginosa, is drug-resistant, making it difficult to treat and potentially leading to severe complications and even death in some cases.
How can an artificial tears injury attorney help?
An EzriCare artificial tears injury attorney can provide legal assistance and guidance to individuals who have suffered injuries or health complications due to contaminated artificial tears. You can contact an attorney for a free case evaluation to discuss legal representation and compensation recovery.

Table of Contents
Millions of Americans suffer from dry eyes. The condition can not only cause inflammation and pain but cause eye damage from an inability to produce enough tears to protect the surface of the eyes. Certain health conditions, medications, and environmental factors are some of the causes of dry eyes.
Symptoms of dry eyes can include blurred vision, light sensitivity, mucus production, the feeling of something in the eye, and stinging or burning pain. These can all impact a person’s comfort and quality of life.
With such a large population affected by dry eyes and possible health complications, treating the condition is important. Though some patients require prescription eye drops and even surgery, over-the-counter artificial tears are a popular treatment option for dry eyes. For many, they’re a cost-effective way to lubricate eyes and stop symptoms altogether.
But now, a rising number of people across the country are experiencing mild to fatal bacterial eye infections linked to using contaminated eye drops. The outbreak has been connected to EzriCare Artificial Tears and Delsam Pharma’s Artificial Tears, with nearly 60 confirmed cases in 13 states.
These over-the-counter products are manufactured by Global Pharma Healthcare Private Limited and are mainly sold online through retailers like Amazon. The company issued a voluntary recall of artificial tears eye drops due to possible contamination, with an additional recall of Delsam Pharma’s Artificial Eye Ointment weeks later.
As a result of the rare bacterial infections, the Centers for Disease Control and Prevention (CDC) and the U.S. Federal Food and Drug Administration (FDA) are working with state and local health services to investigate the cause. So far, laboratory testing of opened, multidose bottles of EzriCare artificial tears has revealed the presence of the same bacteria involved in the outbreak.
Health Risks of Contaminated Artificial Tears
The bacteria involved in the outbreak among artificial tears consumers is Pseudomonas aeruginosa, and this particular strain has never been seen before in the United States. It’s drug-resistant, meaning the antibiotics used to treat bacterial eye infections of this kind are ineffective.
Once the CDC associated artificial tears with Pseudomonas aeruginosa, they gave recommendations for consumers and healthcare professionals about stopping usage and what to do if symptoms occur. They’ve advised anyone who uses or used Global Pharma’s artificial tears and has symptoms of an eye infection to seek medical attention. These symptoms include:
- Yellow, green, or clear discharge from the eye
- Eye pain or discomfort
- Redness of the eye or eyelid
- Feeling of something in your eye
- Increased sensitivity to light
- Blurry vision
The most serious health risk from contaminated artificial tears is that the infection can spread to the blood, which was the cause of the one fatality. Permanent vision loss can occur; in some cases, eye surgery, such as a corneal transplant, may be required.
But Pseudomonas aeruginosa can also affect other areas of the body, such as the respiratory system, and additional complications may arise. While not everyone infected with a drug-resistant bacterial eye infection will experience permanent health issues, it can be serious and cause future medical complications.
If you used EzriCare or Delsam Pharma’s artificial tears, be vigilant. And if you used the artificial tear ointment made by Global Pharma, it’s essential to know the company has recalled batches of that product as well. 
Contaminated Eye Drops Investigation by the CDC & FDA
Between May 2022 and the end of January 2023, 55 patients were diagnosed with Pseudomonas aeruginosa. Laboratory testing by the CDC confirmed the presence of the same strain of bacteria in opened bottles of EzriCare artificial tears. Testing consisted of bottles from consumers both with and without infections.
The FDA discovered the rare bacterial infection outbreak in December 2022 and later linked it to artificial tears in January 2023. Of the confirmed cases, the CDC investigation revealed 38 are connected to four health facility clusters in the U.S.
As of February 21, 2023, 58 people in 13 states had been diagnosed with Pseudomonas aeruginosa. Most patients reported using artificial tears; the most common brand was EzriCare. They suffered mild to severe symptoms, and 16 patients were hospitalized. Unfortunately, these bacterial infections have resulted in five cases of vision loss, with a patient who required surgery to remove an eyeball. The infection was even fatal in one case.
It’s not yet known whether the eye drops were contaminated during the manufacturing process. Further testing of unopened bottles of artificial tears is underway to determine where and how the bacteria developed.
EzriCare Artificial Tears Recalls and Warnings
Over-the-counter eye drops do not need FDA approval to be sold in the U.S. But with confirmed bacteria in opened bottles of EzriCare eye drops, there are clear dangers to consumers. After these findings, the FDA warned consumers not to purchase or use EzriCare artificial tears and Delsam Pharma’s artificial tears at the beginning of February 2023. Consumers and healthcare practitioners were told to immediately discontinue using EzriCare eye drops and monitor for signs and symptoms.
Artificial tears by EzriCare and Delsam Pharma were recalled following the warnings issued by the CDC and FDA. But a few weeks later, the FDA issued another warning about a third eye product manufactured by Global Pharma and recommended a recall of eye ointment cream. They did so the next day and advised anyone with an affected batch of the recalled cream to discard it. So far, the company hasn’t received any reports of the eye ointment causing infection, but the FDA noted permanent blindness could occur if consumers suffer an eye infection from using the product.
Global Pharma Import Alert
In order to ensure the quality of drug products in the United States, the FDA has regulations of Current Good Manufacturing Practice (CGMP) that drug manufacturers must adhere to. These regulations include minimum requirements for the methods, facilities, and controls used for manufacturing, processing, and packing products. The CGMP protects practitioners who recommend the use of and consumers who purchase over-the-counter drug products.
Following the discovery of contaminated artificial tears by EzriCare and recall recommendations, the U.S. Food and Drug Administration also placed Global Pharma Healthcare Private Limited on import alert. The alert prevents their products from entering the country. The FDA did this due to the company’s failure to comply with CGMP requirements and “for providing an inadequate response to a records request.”
Two violations of the Current Good Manufacturing Practice include a lack of microbial testing and the manufacturing and distribution of a multidose bottle that doesn’t contain an adequate preservative. The absence of a preservative puts the product at risk of bacterial growth once the eye drops are opened, ultimately risking the health of consumers. 
Artificial Tears Lawsuits
There are dozens of people who have or have had a dangerous bacterial infection linked to contaminated artificial tears, but doctors expect more infections are on the horizon. Why? They’re worried that consumers are still using recalled eye drops and ointment, which could lead to an influx of patients with a drug-resistant bacterial infection in the coming weeks and months.
Permanent vision loss and other health complications can arise from those suffering from Pseudomonas aeruginosa infections. And with this particular strain being resistant to antibiotics, the effects of an eye infection from using artificial tears can be devastating and lifelong.
Some consumers who suffer from the bacterial infection will or have already undergone surgery, and others have permanently lost their vision. In the worst case, Pseudomonas aeruginosa caused the death of a victim.
As the CDC and FDA investigation continues and more is learned about the bacterial outbreak, patients who use or have used artificial tears and contract an eye infection should seek legal guidance – after seeing a doctor. Claims have already begun, with the first EzriCare artificial tears lawsuit filed on February 9 in the U.S. District Court for the Middle District of Florida. A handful of other artificial tears lawsuits have followed, but many more are expected.
The plaintiff in the first claim, a 60-year-old woman, used EzriCare artificial tears and developed an infection weeks later. After antibiotics didn’t clear up the infection, it caused complications that led to surgery, and she’s still experiencing symptoms. The lawsuit names both Global Pharma and Walmart as defendants.
Another lawsuit alleges EzriCare eye drops caused the plaintiff’s partial blindness and there may be future medical issues from the bacterial eye infection. A third artificial tears lawsuit claims the infection that spread to the plaintiff’s torso, abdomen, and back was caused by contaminated eye drops and that she’s still suffering from complications. This lawsuit cites product liability, breach of warranty, negligence, and negligence per se.
In addition to personal injury claims, a class action lawsuit for consumer fraud has also been filed. It aims to provide refunds to consumers who purchased the recalled products. The class action states the manufacturer and distributors knew or should have known the artificial tears were contaminated but failed to disclose it to the public and marketed the product as safe.
Why Contact an Artificial Tears Injury Attorney
A personal injury lawsuit could help provide compensation for those affected by contaminated artificial tears who face an uncertain future and have already suffered a range of damages. Lawsuits of this kind can provide victims with much-needed financial support to manage the effects of bacterial eye infection, cover lost wages and pain and suffering, among other damages.
If you’ve suffered an eye infection after using EzriCare or Delsam Pharma’s artificial tears or ointment, the first step is to seek medical attention. Consumers and healthcare professionals with patients who have experienced adverse effects after using artificial tears are encouraged to voluntarily report it to the FDA’s Safety Information and Adverse Reporting Program.
After your medical needs are addressed, contact an artificial tears injury attorney to discuss your legal options. You may be entitled to significant compensation for the effects of your condition, and negligent companies should be held responsible for their role in harming consumers.